Releases Details
ARS Pharmaceuticals announces neffy® meets primary endpoints and shows rapid symptom control in Phase 2 urticaria clinical study
- neffy demonstrated statistically significant and clinically meaningful improvement in pruritus, hives, body surface area and erythema in treatment-resistant chronic spontaneous urticaria patients, a skin disorder that causes itchy hives and/or angioedema
- Data supports continued development of neffy in urticaria with planned outpatient study in acute flares to initiate during 2024, with pivotal study to potentially initiate in 2025
These data for neffy are being presented in an oral presentation today,
“The Phase 2 results in this highly refractory patient population are impressive and encouraging as they indicate neffy could be a game-changing therapeutic advance in the treatment of urticaria,” says
Design of neffy efficacy study in urticaria
The EPI-U01 study was a randomized, placebo-controlled, cross-over study evaluating the safety and efficacy of epinephrine nasal spray in patients with chronic spontaneous urticaria (CSU) treated with chronic medications who were still experiencing flares. This oral presentation includes data analysis from 18 adult patients as of the cut-off date who enrolled in this study and returned to the clinical site while experiencing a flare, where they were randomized to receive either a single treatment of 1 mg or 2 mg neffy or placebo in a crossover design.
All subjects had flares with pruritus and hives scores greater than or equal to 2 on a 3 point severity scale, despite all patients having been treated with antihistamines or Xolair.
Results from neffy efficacy study in urticaria
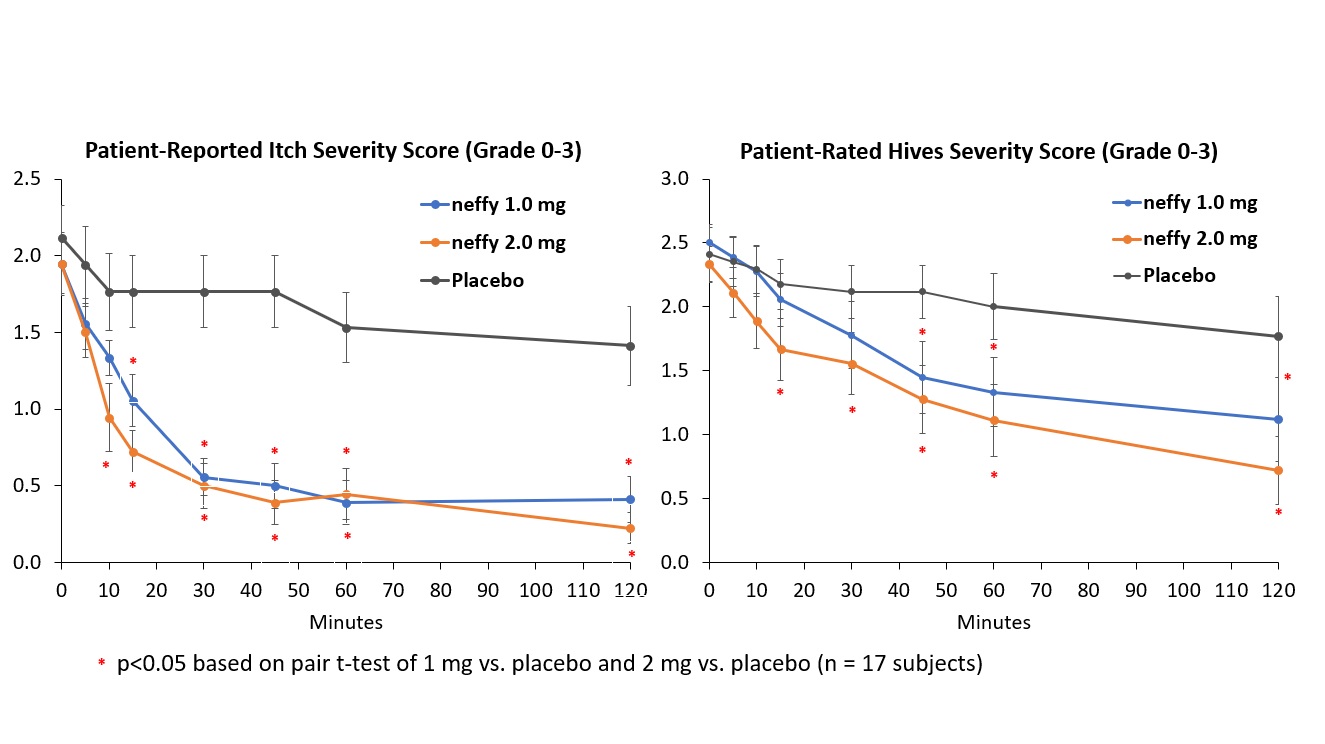
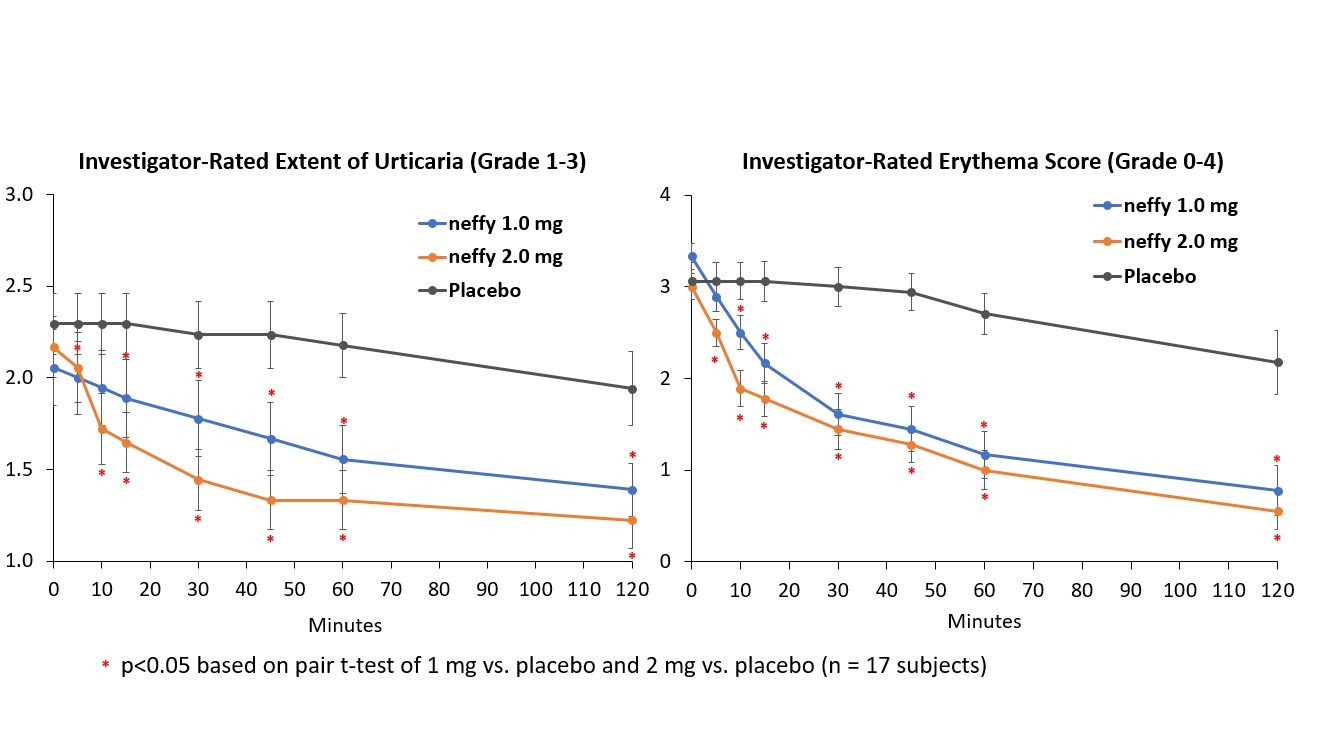
There was no meaningful difference in efficacy on patient-reported itch severity score, patient-reported hives severity score, investigator-related extent of urticaria, or investigator-related erythema score between 1 mg and 2 mg neffy doses, indicating that the 1 mg dose may be sufficient to activate the beta-2 adrenergic receptors responsible for stopping the mast cell degranulation and allergic mediator release that leads to an urticaria flare.
neffy was well-tolerated, with adverse events reported in 8 subjects, which were all mild or moderate in severity. The most common adverse event reported was nasal discomfort in 5 subjects. There were no serious adverse events.
“This study adds to the wealth of data supporting the efficacy and safety of neffy in the treatment of type I allergic reactions,” said
Unmet need in urticaria and role of neffy
Urticaria is a skin disorder driven by mast cell degranulation and histamine release, which causes itchy wheals (hives) and angioedema, or both. In
Antihistamines are the first-line therapy for treatment of urticaria, but 20 to 25% of acute urticaria cases1, and 50% of chronic urticaria cases3 are non-responsive to antihistamines. These non-responsive patients on stable therapy regimens can experience exacerbations or flares several times a year among acute cases, and even several times a week4, including up to 3 or 4 emergency room visits, among chronic urticaria cases.5 Angioedema is also a co-occurring symptom in about 33 to 67% of these patients.6 There are currently no approved community use treatments for acute flares experienced by urticaria patients on chronic regimens of antihistamines. neffy may provide episodic symptomatic relief of these acute flares or exacerbations to improve the quality of life of urticaria patients. Patients would have the option to quickly resolve exacerbations or flares at home without escalating to chronic use of systemic biologics that may have more serious side effects and benefit-risk considerations, or having to visit the emergency room for further treatment.
Next steps for neffy development in urticaria
ARS Pharma plans to initiate a placebo-controlled outpatient urticaria study in patients treated with antihistamines who experience frequent acute flares later in 2024 followed by the potential initiation of a single pivotal efficacy study in 2025. This would follow the anticipated FDA approval of neffy for allergic reactions (Type I) including anaphylaxis in the second half of 2024.
About Type I Allergic Reactions including Anaphylaxis
Type I severe allergic reactions are serious and potentially life-threatening events that can occur within minutes of exposure to an allergen and require immediate treatment with epinephrine, the only FDA-approved medication for these reactions. While epinephrine autoinjectors have been shown to be highly effective, there are well published limitations that result in many patients and caregivers delaying or not administering treatment in an emergency situation. These limitations include fear of the needle, lack of portability, needle-related safety concerns, lack of reliability, and complexity of the devices. There are approximately 40 million people in
About ARS Pharmaceuticals, Inc.
ARS Pharma is a biopharmaceutical company dedicated to empowering at-risk patients and caregivers to better protect themselves from severe allergic reactions that could lead to anaphylaxis. The Company is developing neffy® (previously referred to as ARS-1), an intranasal epinephrine product in clinical development for patients and their caregivers with Type I allergic reactions including food, medications and insect bites that could lead to life-threatening anaphylaxis. For more information, visit www.ars-pharma.com.
Forward-Looking Statements
Statements in this press release that are not purely historical in nature are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These statements include, but are not limited to ARS Pharma’s planned urticaria outpatient study and the timing thereof; the potential pivotal study and the timing thereof; neffy potentially being a game-changing therapeutic advance in the treatment of urticaria and offering patients a treatment option to address existing gaps in the efficacy of currently available antihistamines or biologics; 1 mg dose of neffy potentially being sufficient to activate the beta-2 adrenergic receptors; the potential approval of neffy and the timing thereof; and other statements that are not historical fact. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as “anticipate,” “could,” “indicate,” “may,” “plans,” “will,” “potential” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon ARS Pharma’s current expectations and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include, without limitation, the ability to obtain and maintain regulatory approval for neffy; the results of the clinical trials may not support the approval of neffy; results from clinical trials may not be indicative of results that may be observed in the future; potential safety and other complications from neffy; the labelling for neffy, if approved; ARS Pharma’s ability to protect its intellectual property position; and the impact of government laws and regulations. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” in ARS Pharma’s Quarterly Report on Form 10-Q for the quarter ended
The forward-looking statements included in this press release are made only as of the date hereof. ARS Pharma assumes no obligation and does not intend to update these forward-looking statements, except as required by law.
ARS Media Contacts:
Laura O’Neill
Laura.oneill@finnpartners.com
ARS Investor Contacts:
justinc@ars-pharma.com
References:
1
2 Kolkhir,P. et. al. Urticaria. Nat Rev Dis Primers 8, 61 (2022)
3 Seo JH & Kwon JW. Korean J Intern Med 2019; 34(2):418-425,
4 Raciborski F, et al. Postepy Dermatol Allerg 2018; 35(1):67-73
5 Barniol C et al. Annals of Emergency Medicine 2018; 71(1): 125-131
6 Sussman G et al. Allergy 2018; 73(8): 1724-1734.
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/f7f1b49f-1a9d-4ba8-a389-643fc29729be
https://www.globenewswire.com/NewsRoom/AttachmentNg/b3d077c2-eb70-4da8-b291-326bb965da92

ARS Pharmaceuticals, Inc.