UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported)
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
| |
||
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code:
Not Applicable
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligations of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01 | Regulation FD Disclosure. |
On May 16, 2023, ARS Pharmaceuticals, Inc. released a revised corporate presentation which it made available on its website. A copy of the corporate presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K.
The information under this Item 7.01 of this Current Report on 8-K, including Exhibit 99.1, is furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information shall not be deemed incorporated by reference into any other filing with the Securities and Exchange Commission made by the Company, whether made before or after today’s date, regardless of any general incorporation language in such filing, except as shall be expressly set forth by specific references in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. |
Description | |
| 99.1 | Corporate Presentation | |
| 104 | Cover Page of Interactive Data File (embedded within the Inline XBRL document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: May 16, 2023 | ARS Pharmaceuticals, Inc. | |||||
| By: | /s/ Richard Lowenthal | |||||
| Name: | Richard Lowenthal, M.S., MBA | |||||
| Title: | President and Chief Executive Officer | |||||

THE FIRST NO-NEEDLE, NO-INJECTION SOLUTION for Type I Allergic Reactions Q2 2023 Exhibit 99.1

Forward-looking statements This presentation contains forward-looking statements which include, but are not limited to, statements regarding the design and potential benefits of neffy; the anticipated Prescription Drug User Fee Act (PDUFA) date for neffy; the timing of regulatory approval for and the commercial launch of neffy, if approved; ARS Pharma’s commercialization strategy; the potential market opportunity for neffy; the projected growth thereof and neffy’s ability to capture and grow that market; ARS Pharma’s expected competitive position; ARS Pharma’s potential to become the standard in treatment and transform the treatment of allergic reactions; the likelihood of neffy attaining favorable coverage; the expected intellectual property protection for neffy; and any statements of assumptions underlying any of the foregoing. These forward-looking statements are subject to the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. ARS Pharma’s expectations and beliefs regarding these matters may not materialize. Actual outcomes and results may differ materially from those contemplated by these forward-looking statements as a result of uncertainties, risks, and changes in circumstances, including but not limited to risks and uncertainties related to: the ability to obtain and maintain regulatory approval for neffy; results from clinical trials may not be indicative of results that may be observed in the future; the FDA advisory committee’s decision should not be relied on as an indication that neffy will ultimately be approved; the FDA is not bound by decision of its advisory committee or any of its recommendations and there are a number of instances where the FDA has voted against the recommendations of advisory committees; potential safety and other complications from neffy; the labelling for neffy, if approved; the scope, progress and expansion of developing and commercializing neffy; the size and growth of the market therefor and the rate and degree of market acceptance thereof vis-à-vis intramuscular injectable products; the ARS Pharma’s ability to protect its intellectual property position; the impact of health epidemics or pandemics on ARS Pharma’s business and the actions ARS Pharma may take in response thereto; and the impact of government laws and regulations. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” in the company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2023, filed with the Securities and Exchange Commission (“SEC”) on May 15, 2023. This and other documents ARS files with the SEC can also be accessed on ARS’s web page at ir.ars-pharma.com by clicking on the link “Financials & Filings.” The forward-looking statements included in this presentation are made only as of the date hereof. ARS Pharma does not assume any obligation and does not intend to update these forward-looking statements, except as required by law.

Potential to Transform the Treatment of Type I Allergic Reactions neffy®: first “no needle, no injection” solution for Type I allergic reactions to address an unmet market need Registration program demonstrates comparable PK and PD, without risk of needle-related safety concerns, fear and hesitation Rapid and statistically significant response on PD surrogates for efficacy (SBP, HR) observed even 1 minute after dosing with neffy vs. injection Significant opportunity to disrupt current epinephrine injectables market Mid-2023 PDUFA anticipated; FDA AdCom supports favorable benefit-risk assessment of neffy Potential multi-billion-dollar market driven by HCP and consumer preference and adoption NCE-like IP exclusivity potential until at least 2038 $264.5 million in cash and securities as of 3/31/2023

Proven leadership team with track record developing and commercializing intranasal and consumer-driven medicines Richard Lowenthal, M.S. Chief Executive Officer, Co-Founder Led FDA approvals for multiple nasal spray products 25+ years of experience Robert Bell, Ph.D. Chief Scientific Officer, Co-Founder 30+ years of senior R&D leadership experience including Barr and Somerset Sarina Tanimoto, M.D. Chief Medical Officer, Co-Founder Led FDA approvals for multiple nasal spray products 20+ years of experience Eric Karas Chief Commercial Officer Led Narcan® commercial ops at Emergent/Adapt, and Auxilium specialty 25+ years of experience Harris Kaplan EVP, Commercial Strategy 40+ years of commercial strategy across more than 125 product launches Dan Relovsky SVP, Marketing 30+ years of marketing, sales and operational experience across specialty and consumer markets Brian Dorsey Chief Operating Officer 25+ years of R&D experience as including multiple head of R&D roles including Pernix, Apricus and Somaxon Kathy Scott Chief Financial Officer 30+ years of finance experience with multiple CFO roles including Neurana, Recros and Oncternal Justin Chakma Chief Business Officer 10+ years of M&A, licensing, financing and strategy experience including Celgene, Receptos and Auspex Alex Fitzpatrick Chief Legal Officer 30+ years of legal experience with multiple GC roles including Evofem, Kyriba, Verenium, Blackbaud

Top-tier board of directors Pratik Shah, Ph.D. Chairman of Board of Directors Executive Chairman at Design, Former Chairman of Synthorx (acq. $2.5B), Former CEO at Auspex (acq. $3.5B) Peter Kolchinsky, Ph.D. Managing Partner and Founder at RA Capital Rajeev Dadoo, Ph.D. Managing Partner at SR One Richard Lowenthal, M.S. Chief Executive Officer, Co-Founder Led FDA approvals for multiple nasal spray products 25+ years of experience Brent Saunders Chairman at The Beauty Health Co., Former CEO of Allergan (acq. $63B), Actavis, Forest Labs, and Bausch + Lomb (acq. $8.7B) Michael Kelly Former President, US Operations at Adapt (acq. $735M), CEO at Covis (acq. $1.2B), founder at Azur Jonathan Leff Partner at Deerfield Management Chairman of Deerfield Institute Philip Schneider Former CFO at IDEC, former Board member at Arena (acq. $6.7B), Auspex (acq. $3.5B), GenProbe (acq. $3.7B) Laura Shawver, Ph.D. CEO at Capstan, former CEO at Silverback, Synthorx (acq. $2.5B) Peter Thompson, M.D. Private Equity Partner at Orbimed Saqib Islam, J.D. CEO of Springworks, former CBO at Moderna and EVP at Alexion

Type I allergic reactions: a life-threatening hypersensitivity reaction Carrillo-Martin et al. J Allergy Clin Immunol Pract (2020), BlueCross BlueShield of America. Childhood Allergies in America (2018) Images Reproduced with permission from Allergy & Anaphylaxis Australia Caused by exposure to a specific allergen, most commonly food, venom, drugs ~25 to 40 million people in US with systemic Type I allergic reaction to allergens (e.g., 2+ organ systems involved) 10+ million people with other Type I allergy indications (e.g. urticaria flares, asthma exacerbations) Significant co-morbidities and symptomatic impact on patient quality of life More than half a million1 ER visits each year due to systemic Type I allergic reactions, costing an average of $1600+ per visit2

Epinephrine recognized as the only first-line therapy by allergy society treatment guidelines1, but… Epinephrine is effective, but significant device limitations exist Anaphylaxis – a 2020 practice parameter update, systematic review and Grading Recommendations, Assessment, Development and Evaluation (GRADE) analysis Apprehension to dose due to needle Lack of portability Reluctance to use in public Safety concerns: lacerations, caregiver self-injection, blood vessel hits Lack of reliability Not user friendly 7 fatalities and 35 hospitalizations reported due to failures

Early intervention with epinephrine is critical in a Type I allergic reaction FIRST 15 MINUTES TYPE I SEVERE ALLERGIC REACTION ANTIGEN EXPOSURE 1. Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. Resuscitation Council (UK); 2016, 2. JF Philips et al. Allergy Asthma Proc (2011), 3. JT Fleming et al. J Allergy Clin Immunol Pract (2014), 4. E. Andrew et al. Prehospital Emergency Care (2018), 5. Data on file from ARS market research SERIOUS PATIENT DISCOMFORT HIGHER RISK OF HOSPITALIZATION AND DISEASE PROGRESSION2,3,4 30 MINUTES ANAPHYLAXIS 15 TO 30 MINUTES LIKELIHOOD OF LIFE-THREATENING REACTION Sudden drop in blood pressure leads to anaphylactic shock and cardiovascular failure Airways narrow blocking breathing, leading to loss of consciousness Possible death Time to respiratory arrest or shock1 FOOD: 30–35 minutes INSECT STINGS: 10–15 minutes DRUGS: <10 minutes Hypotension, dizziness, faintness Rhinitis, watery red eyes Rashes, itching (urticaria) Rapid swelling (angioedema) including lips, tongue, throat Bronchospasm, difficulty breathing, wheezing Abdominal and chest pain, vomiting Up to 18 minutes average wait to dose epinephrine5 among the ~50% who have injection available and are willing to inject themselves REACTION PROGRESSION

FAILURE OF TREATMENT 23 - 35%4 fail to dose correctly Limitations of injection lead to hesitation and decreased or ineffective usage neffy may address these limitations to transform the treatment paradigm Rapid administration without a needle No risk of needle-related injuries; lacerations2 or cardiotoxic blood vessel injections Less hesitation to dose NO NEEDLE NO INJECTION SMALL Fits in your pocket; can carry more than 1 ~10% of cases require multiple doses of epinephrine1 EASIER AND MORE CONSISTENT DOSING 0% critical dosing errors in registration self-administration study Low 2 mg dose of epinephrine achieves comparable PK without overexposure risk RELIABLE 99.999% delivery of effective dose in reliability testing; no inhalation required Stability data up to 24 months, including at high temperature for up to 3 months DELAY IN TREATMENT ~40 - 60%2 of patients delay NO TREATMENT AVAILABLE ~50% of patients carry1 (<20% carry two) REFUSAL OF TREATMENT ~25% - 50%1, 3, 5 do not administer 3 4 2 1 neffy® SOLUTIONS PROBLEM Only 10% - 20% of Rx filled or used as indicated6 Warren et al. Ann Allergy Asthma Immunol (2018), Data on file from ARS market research, Brooks et al. Ann Allergy Asthma Immunol (2017), El Turki et al. Emerg Med J (2017), Asthma and Allergy Foundation of America Patient Survey Report (2019), Company estimates based on prior references (1) through (5) and IQVIA data Demonstrated PK/PD comparable to injection with PD response observed 1 min after dosing 3 4 2 1

neffy Designed for Needle-free, Easier Carriage

Approved injection products have a range of PK profiles, but are all deemed efficacious (no known difference across products) 0.3 mg IM (needle & syringe) is considered to be the gold standard, and autoinjectors were approved based on literature support from 0.3 mg IM for efficacy and safety1, 2 Autoinjectors are a variable mix of IV, SC or IM dosing depending on technique All approved products have indistinguishable clinical effect and time to observed clinical benefit All products approved without any PK or PD data required Treatment Source N Mean Study Cmax (pg/mL) Median or Mean Study Tmax (min) Study Tmax Range (min) EpiPen 0.3 mg Literature and ARS 507 288 – 869 5 – 40 1 – 240 IM 0.3 mg Literature and ARS 381 209 – 489 30 to 60 3 – 360 Auvi-Q 0.3 mg Literature 67 486 20 5 – 60 Symjepi 0.3 mg ARS data 88 337 – 438 30 4 – 240 SC 0.3 mg ARS 36 246 45 4 – 180 Total Range 209 to 869 5 to 60 1 to 360

Differences in PK (including tmax - time to max concentration) do not translate to any meaningful difference in efficacy among injection products Analysis of 12 studies with 100% autoinjector (≥ 80% EpiPen) or 100% IM-needle-and-syringe use in community or emergency room or hospital setting, respectively1 Differences in PK profile across products do not impact efficacy based on need for repeat dosing to resolve symptoms Cases in emergency room or hospital settings are typically more severe or advanced (where IM is administered) than those in a community setting, but still no difference in efficacy is observed with IM vs. auto-injector Autoinjector reactions n = 799a IM Needle-in-Syringe reactions n = 570 Treated Reactions Requiring Second Epinephrine Dose (%) ~90% resolution on first dose a. 79.6% of the autoinjector treated reactions are specifically identified occurring with EpiPen
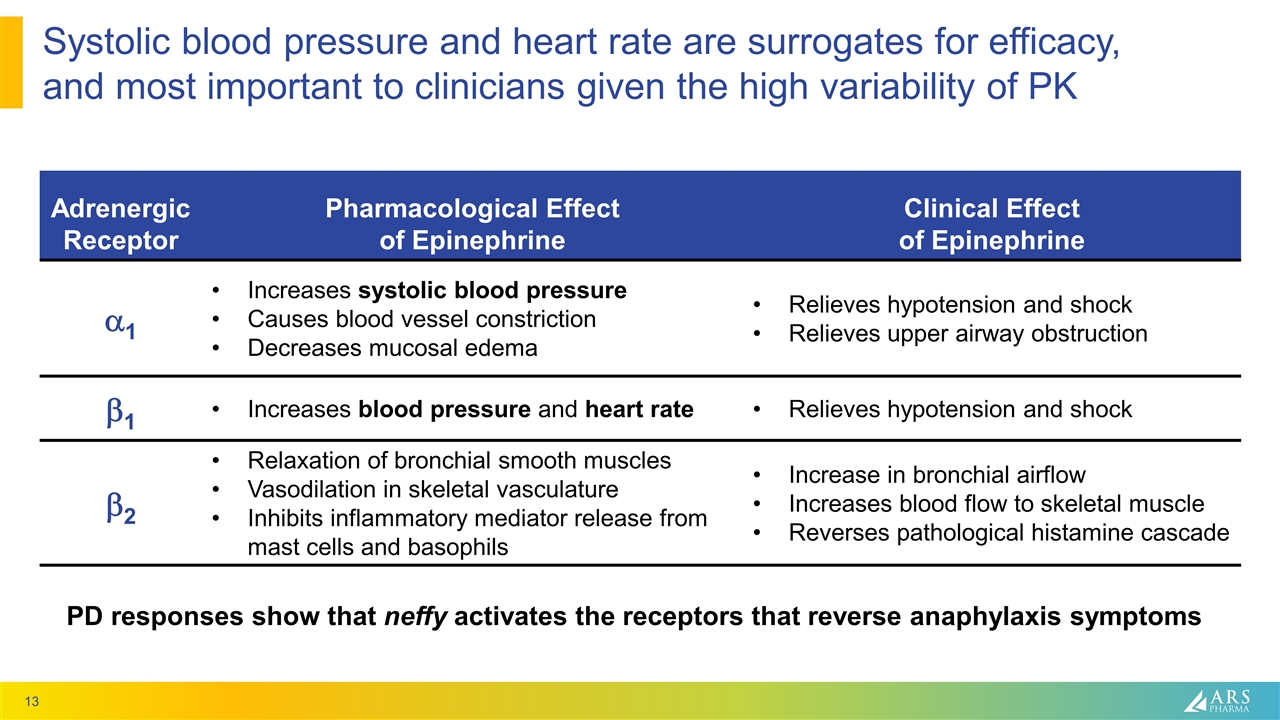
Systolic blood pressure and heart rate are surrogates for efficacy, and most important to clinicians given the high variability of PK Adrenergic Receptor Pharmacological Effect of Epinephrine Clinical Effect of Epinephrine a1 Increases systolic blood pressure Causes blood vessel constriction Decreases mucosal edema Relieves hypotension and shock Relieves upper airway obstruction b1 Increases blood pressure and heart rate Relieves hypotension and shock b2 Relaxation of bronchial smooth muscles Vasodilation in skeletal vasculature Inhibits inflammatory mediator release from mast cells and basophils Increase in bronchial airflow Increases blood flow to skeletal muscle Reverses pathological histamine cascade PD responses show that neffy activates the receptors that reverse anaphylaxis symptoms

neffy clinical program under NDA review; FDA Advisory Committee voted that data supports favorable benefit-risk for allergic reactions (type I) Target PDUFA action date anticipated in mid-2023 FDA Advisory Committee / PADAC (May 11) voted 16:6 and 17:5 in favor of adults and children <18 years of age and ≥30 kg that available data support a favorable benefit-risk assessment FDA confirmed three primary registration studies required for neffy approval EPI-17: Self-administration in Type I allergy patients (n=42) EPI-15: Single dose and twice dosing in healthy volunteers (n=42) EPI-16: Nasal challenge in allergic rhinitis patients (n=36) neffy meets the endpoints discussed with FDA in completed clinical studies* Criteria is comparability to epinephrine injection products: PD (SBP, HR approved products) and PK bracketed (exposures IM/SC for efficacy, < EpiPen for safety) IM needle & syringe is the gold standard and reference-listed drug Primary outcomes for all trials: PD (SBP, HR) and PK (bioavailability) EPI-10 pediatric trial interim data included in NDA submission, FDA requested Data in subjects aged 4 to 18 (single-arm, non-comparative expected in 2022) to support pediatric labeling

Notable PD response observed with neffy even at 1 minute after dosing, and comparable to or significantly higher than 0.3 mg IM injection Mean Change HR (BPM) Time (Minutes) Heart Rate Response Mean Change SBP (mmHg) Time (Minutes) Systolic Blood Pressure Response First PD 1 minute First PD 1 minute neffy 2 mg IM 0.3 mg neffy 2 mg IM 0.3 mg ** Significance level: ** p <0.01, *** p <0.001 **** p <0.0001 **** **** *** ** **** **** **** Epi 53 pg/mL (2 min PK) Epi 53 pg/mL (2 min PK)

PD response is comparable to EpiPen on single dose, with significantly higher response on second dose neffy 2 mg n = 78 EpiPen 0.3 mg n = 77 IM 0.3 mg n = 178 p < 0.0001 p = 0.0787 (ns) Emax (mmHg) 2 mg x 2 neffy (L/R) n = 39 0.3 mg EpiPen n = 78 0.3 mg IM n = 70 p = 0.947 (ns) p = 0.01 Emax (mmHg) p < 0.0001 2 mg x 2 neffy (R/R) n = 39 Single Dose SBP Response Twice Dose SBP Response

neffy meets PK endpoints agreed with FDA in 3 primary studies* Integrated PK data summary for neffy and comparators Plasma Epinephrine Cmax (pg/mL) Median tmax (minutes) Mean Early Partial AUCs 0.3 mg IM (n = 178) 2 mg neffy HCP-admin (n = 78) 0.3 mg EpiPen (n = 77) 0.5 mg IM (n = 123) 2 mg neffy Self-admin (n = 42) 10 45 45 20.5 30 Treatment pAUC (0-20) pAUC (0-45) 0.3 mg IM (n=178) 2,090 (86) 6,290 (61) 2 mg neffy (n=78) HCP-admin 3,610 (84) 11,000 (76) 2 mg neffy (n=42) Self-admin 3,128 (79) 11,006 (63) 0.3 mg EpiPen (n=77) 5,640 (73) 12,000 (53) Treatment AUC0-t 0.5 mg IM (n=123) 2 mg neffy (n=36) 0.3 mg IM (n=178) 43,700 (34) 37,700 (64) 27,900 (39) Mean early partial AUCs bracketed by approved products Overall mean AUC(0-t) bracketed by approved products 0.3 mg IM (n = 178) 2 mg neffy HCP-admin (n = 78) 0.3 mg EpiPen (n = 77) 0.5 mg IM (n = 123) 2 mg neffy Self-admin (n = 42) Lowest Cmax of approved product Highest Cmax of approved product Lowest Tmax of approved product Highest Tmax of approved product Cmax bracketed by approved products Tmax bracketed by approved products Mean values denoted by bolded numbers All data from ARS clinical studies % CV shown in parentheses in tables above

neffy PK is Bracketed by EpiPen Studies (high variability) Treatment Study Reference N Mean Study Cmax (pg/mL) Median Study Tmax (min) EpiPen (0.3 mg) AQST-109 EPIPHAST II Results (2022) 22 869 22 ARS EPI-JP01 Data (2020) 30 676 10 ARS EPI-15 (2022) 35 612 8 Tal et al. EAACI (2022) 12 550 9 ARS EPI-11b Data (2021) 9 537 6 Edwards et al. NDA #201739 (2012) 67 520 10.2 Chen et al. AAAAI (2019) 11 511 5 ARS EPI-12 Data (2021) 36 493 8 ARS EPI-13 Data (2022) 39 490 6 neffy (2.0 mg) ARS EPI-16 data (2022) 36 491 20 ARS integrated analysis (2022) EPI-15/16 78 485 20.5 ARS EPI-15 data (2022) 42 481 30 ARS EPI-17 data (2022) 42 421 30 EpiPen (0.3 mg) Worm et al. Clin Transl Allergy (2020) 12 390 to 530 9 to 30 Turner et al. Clin Exp Allergy (2021) 37 386 40 Amphastar US2021/030502 (2021) 56 364 - 458 7-15 ARS EPI-07 Data (2019) 35 375 24 Dworaczyk et al. AAAAI (2020) 55 308 to 440 10-16 Oppenheimer et al. AAAAI (2022) 10 341 22 ARS EPI-01 Data (2018) 12 333 20 Aquestive R&D Day (2021) 9 300 104 Dworaczyk et al. AAAAI (2021) 25 288 10

Dosing neffy immediately following nasal allergen challenge (worst-case conditions) shows no clinically meaningful impact on PK or PD ~2 to 11% of patients experience nasal symptoms during an allergic reaction1 Congestion accelerates absorption, and rhinorrhea accelerates drainage neffy during moderate to severe congestion and rhinorrhea following nasal allergen challenge in allergic rhinitis patients has significantly higher exposures than IM during early time points when treatment response is observed If no response is observed within 15 minutes, a second dose of epinephrine is given Regardless, PD response after one dose of neffy (with rhinitis) is comparable to injection (no rhinitis) through 60 min despite the systemic inflammation reported to be triggered by allergic rhinitis2 *significant difference (p<0.05) neffy with rhinitis vs. IM * * * * * *

Dosing neffy during congestion/rhinitis due to an upper respiratory tract infection (e.g. cold or flu) has no clinically meaningful impact on PK or PD Mean Epinephrine Plasma Mean Systolic Blood Pressure Change Mean Epinephrine Concentration (pg / mL) ±SE Time (Minutes) Mean SBP Change from Baseline (mmHg) ± SE Time (Minutes) neffy neffy neffy neffy N = 21 URTI / 16 Normal (returned)

neffy well-tolerated across 600+ individuals dosed in clinical program Well-tolerated at all single-doses (0.5 mg to 2 mg) and repeat doses up to 4 mg within 10 minutes Mostly grade 1 events and comparable to injection products Low Pain Scores: recorded by VAS (100mm scale) with mean scores between 5 and 8 out of a score of 100 across studies No irritation based on formal scoring in all studies No serious treatment-related adverse events No risk of needle-related injuries or blood vessel injections Event Frequency EpiPen Symjepi Risk of blood vessel injection during self-administration that could lead to adverse events All data from ARS clinical studies

neffy market exclusivity potential until at least 2038 Issued composition of matter patent (US10,576,156) on Intravail® + epinephrine provides foundational exclusivity blocking any generic products. Method of treatment patents (US11,173,209; US11,191,838) block other alkyl glycosides. Issued method of treatment patent (US10,682,414) blocks any intranasal epinephrine product using a different technology using a low dose (<2.5 mg) PCT patent granted in Europe (EP19751807), UK (GB2583051), Japan (JP6941224), Canada (3088909), Australia (AUS2019217643), Korea (10-2375232), China (2019800010042), with same claims as the US Extensive studies in the lab and clinic completed to develop a proprietary product with expected NCE-like exclusivity EXPECTED LAUNCH FIRST PATENT EXPIRATION (without patent term extension) ADDITIONAL PROTECTION 2023 2038

Commercial Opportunity and Strategy

MULTIPLE LEVERS OF CURRENT MARKET GROWTH Significant existing US market opportunity for neffy penetration CURRENT ~$1 BILLION1 ANNUAL EPINEPHRINE MARKET IS THE IMMEDIATE OPPORTUNITY 1. SEC filings, IQVIA data and ARS payer research data on file ARS market research data on file (n = 75 physicians, n = 150 patients), 3 IQVIA extended unit data ~3.3M Patients have injectable today (~10 million devices)3 ~16M diagnosed and HCP-managed patients with severe type I allergic reactions (claims data) Consistent market growth +5% y/y in the last ~15 years Promotional responsiveness +31% historic lift from Mylan No meaningful promotion today More devices per patient Potential for twice as many neffy devices annually vs. injectables ~2.5M Former patients discontinued or did not fill Rx in last 3 years Up to 40M total type I allergy patients (epidemiology)

Physicians supportive of adopting neffy into practice Would prescribe neffy if their patients asked for it 100% 10 = MAJOR ADVANCE / 1 = NOT AN ADVANCE AT ALL 8.5 out of 10 rating viewed as a major advance in therapy n = 75 Physicians No difference in uptake of neffy by physician specialty

neffy addresses the unmet need and is better aligned with what healthcare providers, patients and parents want 65% to 72% OF THE TIME, PEOPLE WHO USE AN OTC WOULD USE neffy FIRST 69% OF PEOPLE WOULD USE neffy SOONER THAN CURRENT AUTOINJECTOR OF NON-FILLING PATIENTS STATED THEY WOULD ASK THEIR PHYSICIAN ABOUT neffy RX 75% n = 150 Current Users OF PATIENTS EXPECTED TO SWITCH TO neffy ~80% n = 100 Non-fillers
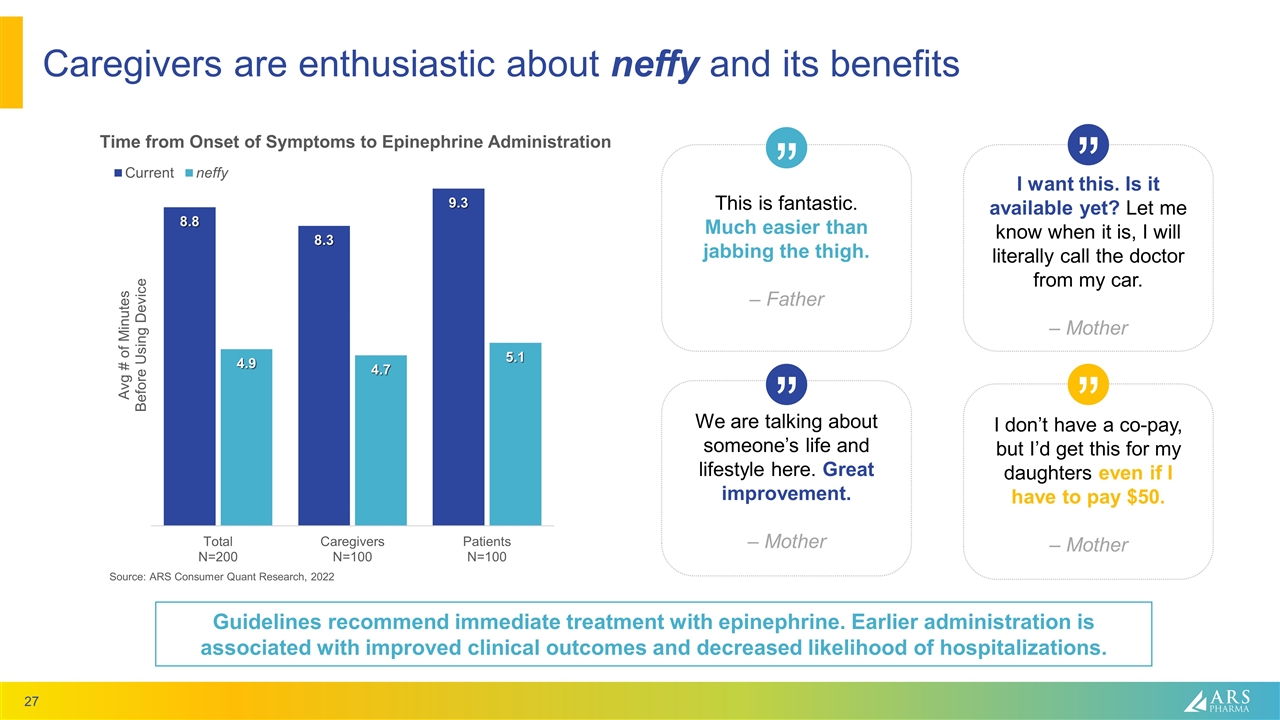
Caregivers are enthusiastic about neffy and its benefits Source: ARS Consumer Quant Research, 2022 This is fantastic. Much easier than jabbing the thigh. – Father We are talking about someone’s life and lifestyle here. Great improvement. – Mother I don’t have a co-pay, but I’d get this for my daughters even if I have to pay $50. – Mother I want this. Is it available yet? Let me know when it is, I will literally call the doctor from my car. – Mother “ “ “ “ Guidelines recommend immediate treatment with epinephrine. Earlier administration is associated with improved clinical outcomes and decreased likelihood of hospitalizations.

Propensity of Current SAR Patients Prescribed a Needle Injector 50% Don’t Keep On Hand2 42% Don’t Use Properly1 By Addressing Needle Injector Deficiencies neffy can Become the Standard in Treatment 60% Delay Treatment3 86% Would Keep On Hand3 neffy Patient Research Shows… 100% Used Device Correctly4 (1) ARS Consumer Quant Research, 2022, (2) Warren et al. Ann Allergy Asthma Immunol (2018), (3) Data on file from ARS market research, (4) ARS human factors studies 45% Faster Treatment Time3 More Likely to Fill Rx3

Payer research supports positive reimbursement environment Key findings from discussions with ~50 decision-makers within the major payers and PBMs: Category is generally not restricted, unlike biologics and orphan disease drugs with high WACs Payers view neffy as a valuable and differentiated treatment option High likelihood of attaining favorable coverage (Tier 2 or 3) for ~80% of lives “This is a game-changer; it really addresses the unmet needs we currently have in this space, specifically the safety and tolerability issues.” – Payer “If this is priced properly, this could be a ‘state-of-the-art therapy’ for patients.” – PBM “Nasal delivery will overcome some negative perceived factors of an injection.” – Payer “There is no value in delaying access to a product like this and nothing to prior authorize (PA). We can’t PA if the patient needs it.” – PBM

Commercial strategy and imperatives Ensure broad and rapid neffy coverage as well as affordable access for patients Change HCP habits and switch prescribing from needle to neffy Drive neffy awareness and new patient growth (into and back into) the market 3 1 2 From needle to neffy: Convert the existing market Bring back patients that are lapsed Bring in patients who should be carrying epinephrine now, but do not carry
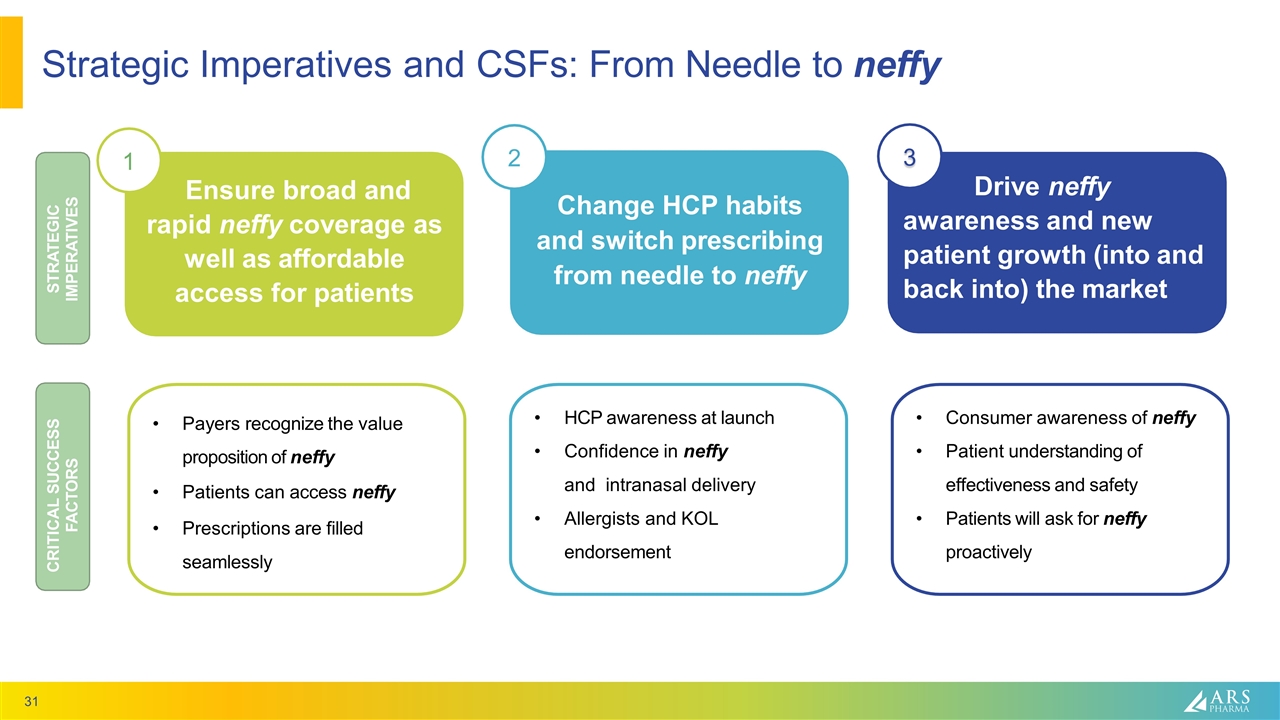
Drive neffy awareness and new patient growth (into and back into) the market Ensure broad and rapid neffy coverage as well as affordable access for patients Strategic Imperatives and CSFs: From Needle to neffy Change HCP habits and switch prescribing from needle to neffy 1 2 3 Payers recognize the value proposition of neffy Patients can access neffy Prescriptions are filled seamlessly HCP awareness at launch Confidence in neffy and intranasal delivery Allergists and KOL endorsement Consumer awareness of neffy Patient understanding of effectiveness and safety Patients will ask for neffy proactively CRITICAL SUCCESS FACTORS STRATEGIC IMPERATIVES

Integrated HCP Promotion to Drive Awareness and Reach with Current Epinephrine Prescribers Representing >40% of Prescriptions* Specialty Salesforce Virtual Salesforce Non-Personal Promotion FTEs ~125 FTEs 40 - 50 Top 50,000 Decile 5 to 10 HCP Reach ~ 15,000 HCPs ~3,000 Top 12,000 HCP promotion will be supported by DTC promotion to drive expansion within the addressable SAR market * Reaching >80% of Prescriptions from Allergists, ENTs, and Pediatricians

neffy is positioned potentially to transform the treatment of serious allergic reactions Less Hesitation to Dose Effective Compact Safe Easy to Use No Needle PK comparable or superior to IM (reference listed drug) and less than EpiPen (upper limit for safety) offers therapeutic exposures and makes it easy to switch No meaningful side effects No needle-related injuries possible No serious safety risks associated with injection devices (needle) No blood vessel injections, no lacerations, no bone injections No needle, no fear, no wait Easier to use and more reliable delivery than with autoinjectors (less chance of failed dosing) Always available with child, parent and caregiver 100% of adults (N=105) successfully dosed in human factors studies

Supplemental Slides

Many patients/caregivers do not administer treatment or delay use during reaction Did not fill or re-fill their Rx in last 3 years1 ~50% do not carry2 Patients (millions) Rx’ed Epinephrine in Last 3 Years1 40-60% delay 9 to 18 min6 25-50% refuse to inject and go to ER2-5 23-35% fail to dose correctly7 ~10%-20% of patients receiving Rx treat as indicated (~2% of overall population) 1. IQVIA Claims Data 2022, 2. Warren 2018, 3. Brooks 2018, 4. Asthma and Allergy Foundation of America 2019, 5. Casale 2022, 6. ARS data presented at AAAAI 2023, 7. El Turki 2017 Approx. 40,000,000 people with serious Type I Allergic Reactions ~5,800,000 people received Rx from a Physician in Last 3 Years

Basis of Approval for Community Use Products Approved community use products include IM and SC dosing (FDA briefing book) Almost all approved without PK data Device Approval Basis Pharmacokinetics (any data including literature) FDA Approved Route and Dose EpiPen® (1987) No PK Data Significant differences (EpiPen vs. IM) only known for ~10 yrs Blood vessel injection risk (IV bolus) known last 5 yrs IM & SC 0.15 & 0.3 mg Twinject® (2003) No PK Data No PK data known to date IM & SC 0.15 & 0.3 mg Adrenaclick® (2003) No PK Data No PK data known to date IM & SC 0.15 & 0.3 mg Auvi-Q® (2012) Single PK Study More rapid PK vs. IM, but slower PK vs. EpiPen (Tmax = 20 min vs 10 min) IM & SC 0.1, 0.15 & 0.3 mg Symjepi® (2017) No PK Data ARS studies show slower PK vs neffy or other autoinjectors IM & SC 0.15 & 0.3 mg Teva EpiPen® (2018) No PK Data None to date; shorter needle and different activation force IM & SC 0.15 & 0.3 mg
